Ammonium Perchlorate Ph In Water
Indicate whether each of the post-obit compounds volition gave an acidic, basic Or neutral solution when dissolved in water: Clear AlL ammonium perchlorate The pH volition be less than sodium hypochlorite The pH will be approximately equal to 7. sodium perchlorate The pH will exist greater than 7 barium nitritc

Related Question
Indicate whether each of the post-obit compounds will gave an acidic, bones O neutral solution when dissolved in h2o: Clear All ammonium nitrate The pH will be less than 7_ barium fluoride The pH will be approximately equal to 7 calcium perchlorate The pH will be greater than potassium iodide
Give-and-take
You must be signed in to hash out.
Video Transcript
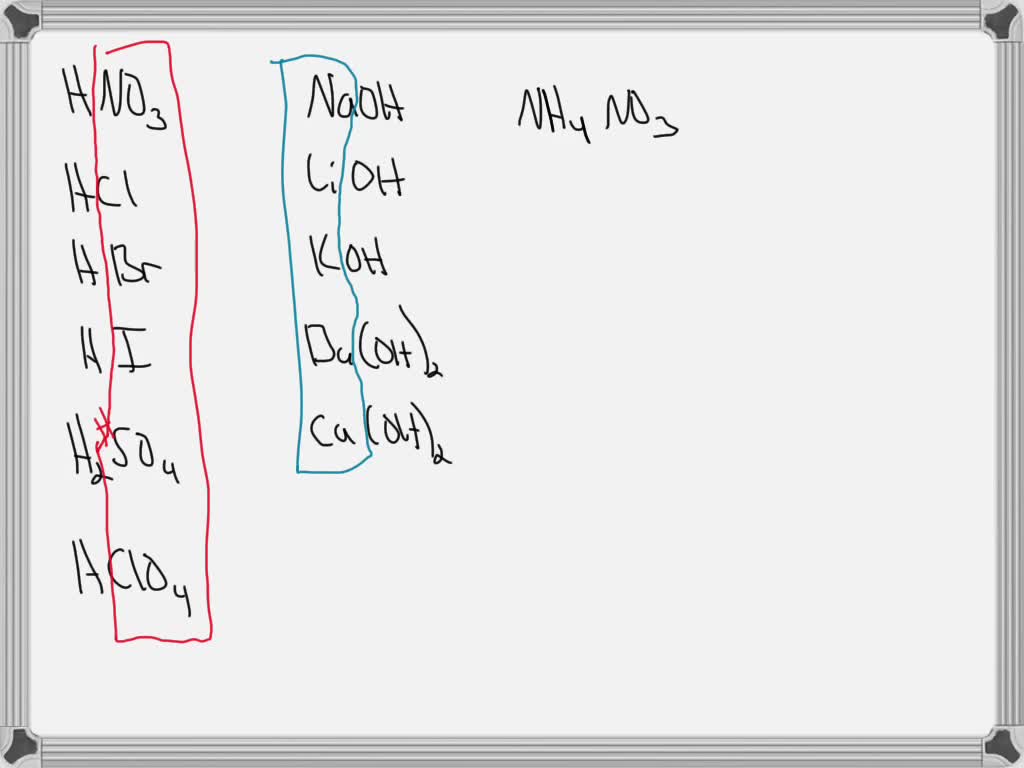
the fundamental to answering this question is to retrieve what your strong avails are in your strong basis. If there is an an eye on that comes from a strong acrid that an ion does not affect the ph if we have a cat eye on that comes from a potent base, so that cat ion does not affect ph And so here are all of the strong acids their corresponding and ions practise not touch on the ph and hither are all the potent bases in a. O. Age, Ally ohh, K O H BAOH ii, C A oh H two. So whatsoever salt that contains these true cat ions, the cat ion won't affect the ph oops, including the N. A. Okay, I'll start over and if assault contains whatever of these ann ions that an ion won't affect the ph this is really going to be H. south. 0. 4. So for the get-go one nosotros have ammonium nitrate. So the nitrate won't affect the ph the ann ions typically desire to brand things basic. True cat ions want to brand things acidic. Then this won't affect ph but the ammonium. Cat ion will cat ions desire to brand things acidic. And then this will exist acidic, Ph will be less than seven. And we've got barium fluoride. The barium won't affect the ph, the fluoride is non hither, it'due south an an ion. So information technology will brand things basic and ph Volition be greater than seven. And so we've got calcium per chlorate. So nosotros've got per chlorate that won't impact the ph we've got calcium that won't impact the ph so this volition be neutral And Ph volition be approximately vii. And for the last 1 nosotros've got potassium iodide. Same thing ph will exist neutral approximately seven. Why? Considering potassium is part of or comes from the strong acid, potassium hydroxide and iodide comes from the strong, so strong based potassium hydroxide. Iodide comes from the strong acrid H I. I hope that helps.
Ammonium Perchlorate Ph In Water,
Source: https://www.numerade.com/ask/question/indicate-whether-each-of-the-following-compounds-will-gave-an-acidic-basic-or-neutral-solution-when-dissolved-in-water-clear-all-ammonium-perchlorate-the-ph-will-be-less-than-sodium-hypochlo-65438/
Posted by: roomdoduchis.blogspot.com



0 Response to "Ammonium Perchlorate Ph In Water"
Post a Comment